Dashboard
- All
-
Following
You must log in
before getting
tailored content. -
Interests
You must log in
before getting
tailored content. - Most liked
- Most viewed

Dr. Sara Borna
Ketamine Haven ClinicDirectory:
Expertise:
At Ketamine Haven Clinic, we offer cutting-edge IV therapy los angeles designed to revitalize your body and mind. Our personalized IV infusions deliver essential vitamins, minerals, and hydration directly into your bloodstream, ensuring maximum absorption and immediate results. Whether you're seeking relief from chronic fatigue, rejuvenation after a long week, or support during recovery from illness or mental health challenges, our treatments are tailored to meet your unique needs.
Experience the transformative effects of our meticulously formulated IV therapies that can boost energy levels, enhance mood stability, and improve overall wellness. Each session is supervised by our knowledgeable medical professionals who assess your individual requirements before administering a customized blend that may include nutrients like Vitamin C for immune support or magnesium for muscle relaxation. These targeted formulations work synergistically to combat deficiencies that can impact both physical health and emotional well-being.
At Ketamine Haven Clinic, we prioritize comfort as much as effectiveness; you can relax in a soothing environment while receiving treatment designed to reinvigorate you from within. With easy scheduling options and flexible service plans, achieving optimal health has never been more accessible. Let us help you unlock your potential with therapeutic IV treatments—your journey towards renewed vitality begins here.
Contact Us:
Phone: 818-817-6542
Email: support@ketaminehaven.com
Website: https://ketaminehaven.com/
view this profile
Top rated Dentist Redondo Beach
Catalina DentalCatalina Dental is your trusted dental implant in redondo beach, committed to delivering top-quality dental services. Our experienced team offers a comprehensive range of treatments, including preventive care, restorative dentistry, and cosmetic procedures. With a focus on personalized care, we strive to create a comfortable and friendly environment for all patients. Take charge of your oral health; visit the dentist today!
view this profile
Directory:
Tags:
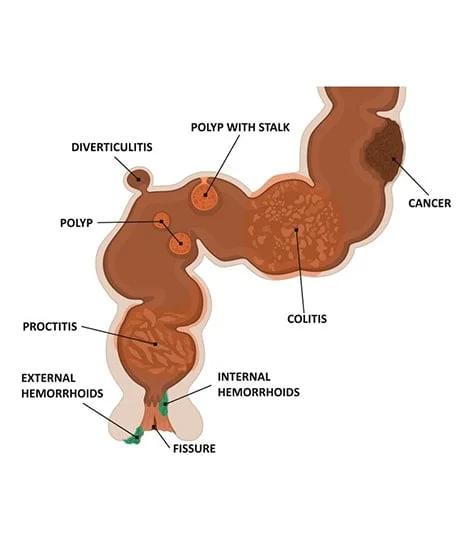
What Is Rectal Bleeding?
Rectal bleeding (medically known as hematochezia) refers to any blood passing from your anus. However, rectal or anal bleeding is commonly assumed to refer to bleeding from the rectum or your colon. It can manifest as blood in your stool, toilet paper, or the toilet bowl. The blood in the stool caused by rectal bleeding from the anus can range from bright red to dark maroon to a dark tarry color.
Rectal bleeding can be a symptom of colorectal or anal cancer, which can be treated if caught early. In addition to a complete physical exam and medical history, colonoscopy is the gold standard for visual evaluation of your colon for an accurate diagnosis. It can detect polyps, mass lesions, abnormalities of the intestine lining, and a variety of other causes of your symptoms.
This condition can also be caused by bleeding hemorrhoids, anal fissures, colitis, or other diagnosable and treatable conditions. Disorders higher up in the digestive tract can also lead to bleeding from the anus.
For these reasons, consulting an experienced gastroenterologist is the first step in determining the underlying causes of rectal bleeding and the best hematochezia treatment options. To perform the proper treatment, we must first correctly diagnose the cause. Internal hemorrhoids and anal fissures are two common causes of rectal bleeding. Colonoscopy, in combination with a thorough physical exam, is the gold standard for visual evaluation of the colon for diagnosis. We can treat these diseases more effectively if we diagnose, detect, and address them early.
Read more: https://www.manhattangastroenterology.com/conditions/rectal-bleeding/
Working Hours:
Monday: 7:30 am - 7:00 pm
Tuesday: 7:30 am - 7:00 pm
Wednesday: 7:30 am - 7:00 pm
Thursday: 7:30 am - 7:00 pm
Friday: 7:30 am - 7:00 pm
Saturday: CLOSED
Sunday: CLOSED
Payment: cash, check, credit cards.
Manhattan Gastroenterology
80 Maiden Ln, Suite 1204B
New York, NY 10038
(646) 813-2095
https://www.manhattangastroenterology.com/gastroenterologist-downtown-financial-district/
Location on the map:
https://maps.app.goo.gl/xXW8HAq2qUFeaX2d8
https://plus.codes/87G7PX4R+RV
Nearby Locations:
Lower East Side | Chinatown | Battery Park City | Soho | Tribeca
10038, 10002, 10003, 10004, 10009, 10012, 10013, 10014
What Is Abdominal Pain?
If you’re suffering from abdominal pain in Manhattan or one of the other boroughs, it can be a mild inconvenience treatable with over-the-counter remedies or a sign of a severe illness that requires immediate attention from a gastroenterologist.
Abdominal pain should always be evaluated with a thorough consultation and examination by a gastroenterologist for an accurate diagnosis and treatment plan, as it may be a symptom of a severe illness or condition. There are multiple causes of stomach pain that should be investigated by a top GI specialist. Abdominal pain can be acute or chronic, and it can result from problems with the stomach, appendix, gallbladder, spleen, bowel, liver, or gynecological issues.
Therefore, it is critical that you see our practice’s doctor for an examination and further investigation to determine the source of your pain. If you’re experiencing abdominal pain in the Upper East Side, your problem deserves to be addressed by a best-in-class gastroenterologist.
Read more:https://www.manhattangastroenterology.com/conditions/abdominal-pain/
Working Hours:
Monday: 7:30 am - 7:00 pm
Tuesday: 7:30 am - 7:00 pm
Wednesday: 7:30 am - 7:00 pm
Thursday: 7:30 am - 7:00 pm
Friday: 7:30 am - 7:00 pm
Saturday: CLOSED
Sunday: CLOSED
Payment: cash, check, credit cards.
Manhattan Gastroenterology
80 Maiden Ln, Suite 1204B
New York, NY 10038
(646) 813-2095
https://www.manhattangastroenterology.com/gastroenterologist-downtown-financial-district/
Location on the map:
https://maps.app.goo.gl/xXW8HAq2qUFeaX2d8
https://plus.codes/87G7PX4R+RV
Nearby Locations:
Lower East Side | Chinatown | Battery Park City | Soho | Tribeca
10038, 10002, 10003, 10004, 10009, 10012, 10013, 10014

Manhattan Gastroenterology (Downtown/Financial District)
GastroenterologyDirectory:
Expertise:
Downtown Manhattan's Financial District GI specialists of Manhattan Gastroenterology offer the most advanced gastroenterology, endoscopy, and colonoscopy ues services. Our gastroenterologists are board-certified by ABIM and are best-in-class gastrointestinal specialists. Well-known in the neighborhood as best GI doctors specializing in preventing, diagnosing, and treating digestive disorders, they deliver highly personalized and comprehensive care. Our gastroenterologist's philosophy regarding the doctor/patient relationship is based on trust and has earned them one of the most respected reputations in New York City. Financial District-based Gastroenterology practice serves the Lower Manhattan/Downtown Manhattan community, including nearby neighborhoods Lower East Side, Tribeca, Chinatown, Battery Park City, and Soho.
At Manhattan Gastroenterology, specialists utilize the latest technological advancements, including the state-of-the-art Quintron Breath Analyzer diagnosis of lactose, fructose, and small intestinal bacterial overgrowth that has been shown to demonstrate effectiveness in the treatment and management of Irritable Bowel Syndrome (IBS). They also offer a minimally invasive, non-surgical laser hemorrhoid treatment called IRC or infrared coagulation that is safe and effective.
Through the integrative approach, they spend the time necessary to analyze and treat difficult digestive issues often overlooked in today's fast-paced healthcare environment.
We offer services such as:
laser treatment of hemorrhoids
colonoscopy procedure
ibs treatment
gerd disease treatment
abdominal pain treatment
For more information about Manhattan Gastroenterology practice or to schedule an appointment, don't hesitate to contact the Gastroenterologist Downtown/Financial District at (646) 813-2095.
Also visit how to find Gastroenterologist near me
Working Hours:
Monday: 7:30 am - 7:00 pm
Tuesday: 7:30 am - 7:00 pm
Wednesday: 7:30 am - 7:00 pm
Thursday: 7:30 am - 7:00 pm
Friday: 7:30 am - 7:00 pm
Saturday: CLOSED
Sunday: CLOSED
Payment: cash, check, credit cards.
Manhattan Gastroenterology
80 Maiden Ln, Suite 1204B
New York, NY 10038
(646) 813-2095
https://www.manhattangastroenterology.com/gastroenterologist-downtown-financial-district/
Location on the map:
https://maps.app.goo.gl/xXW8HAq2qUFeaX2d8
https://plus.codes/87G7PX4R+RV
Nearby Locations:
Lower East Side | Chinatown | Battery Park City | Soho | Tribeca
10038, 10002, 10003, 10004, 10009, 10012, 10013, 10014
Our social links:
https://www.facebook.com/ManhattanGastro/
https://twitter.com/KhodadadiaShawn
https://www.linkedin.com/in/shawn-khodadadian-96b70616b/
https://www.linkedin.com/company/manhattan-gastroenterology/
https://www.instagram.com/manhattangastroenterology/
https://www.youtube.com/channel/UC7fdxh_2KoQBpYGO8vpRlWQ
https://www.flickr.com/people/156351723@N02/
https://shawnkhodadadian.tumblr.com/
https://www.yelp.com/biz/manhattan-gastroenterology-maiden-manhattan
https://www.tiktok.com/@manhattan_gastro
Find us at:
https://www.md.com/doctor/shawn-khodadadian-md
View other locations Manhattan Gastroenterology has been mentioned:
https://citysquares.com/b/manhattan-gastroenterology-25561602
https://www.cylex.us.com/company/manhattan-gastroenterology-37197213.html
http://ezlocal.com/ny/manhattan/gastroenterologist/0917988597
https://www.hotfrog.com/company/daece22a310e9c01dc8db9f63a994ae4
view this profile
5-Star Rated Dentist North Phoenix
North Valley Family DentistDirectory:
Expertise:
North Valley Family Dentist is your premier destination for a top-tier dentist in Phoenix. Our skilled and compassionate team is dedicated to providing exceptional dental services, including dental crowns & bridges, periodontal treatment, sedation dentistry, teeth whitening, root canal therapy, and more . With a commitment to patient comfort and satisfaction, we ensure your smile is in the best hands. Experience the difference and achieve a healthier, more beautiful smile today. Visit our website to learn more!
view this profile

Top-rated Dentistry Northridge
Fuller SmilesDirectory:
Expertise:
Fuller Smiles is your trusted destination for a top-rated Northridge dentist. We are committed to transforming dental experiences into radiant smiles. Our expert team offers comprehensive dental services, including emergency dentistry, Invisalign, IV sedation, pediatric dentistry, cosmetic dentistry, oral surgery, and restorative dentistry. Discover a dental journey marked by excellence and genuine care, where your smile receives the attention it deserves. For more information, Visit our website.
view this profile

Leamington Road Dental Practice
Created by: Priyesh LadOwned by Priyesh and Vina Lad, Leamington Road Dental Practice is a private Dentist Coventry, offering a wide range of treatments to cater to diverse oral health needs. Their mission is simple – to help you achieve and maintain a healthy, beautiful smile that you can be proud of.
Their team of skilled Cosmetic dentist Coventry, dental hygienists, and support staff are dedicated to staying at the forefront of dental technology and techniques to provide you with the most outstanding care possible.
Leamington Road Dental Practice is a modern, well-equipped surgery designed with your comfort in mind.
From dentures to dental implants, the practice offers a range of restorative solutions to restore functionality and aesthetics to your smile. The clinic focuses on providing durable and natural-looking restorations like fillings, veneers, crowns, bridges, and composite bonding tailored to each patient's unique needs.
Additionally, transform your smile with Invisalign Clear Aligners, ensuring a confident and natural-looking result.
Whether you're interested in dental or facial aesthetics treatments like anti-wrinkle injections, Dermal fillers Coventry, and microneedling, Leamington Road Dental Practice has you covered.
In case of toothache, bleeding, or swelling, call the emergency Dentist Coventry at 02476 414 302 for a same-day appointment and pain relief.
go to cluster
Revive Rehab Physiotherapy
Revive Rehab PhysiotherapyRevive Rehab Physiotherapy is a leading physiotherapy and rehabilitation center located in KPHB, Hyderabad. We specialize in neuro rehabilitation, traumatic brain injury recovery, and general physiotherapy services. Our experienced team of therapists is dedicated to providing personalized care to help patients achieve optimal health and mobility.
Phone Number:
+91 9885 982 698
Business Hours:
Monday to Friday: 9:00 AM - 6:00 PM
Saturday: 9:00 AM - 2:00 PM
Sunday: Closed
view this profile

Cindy Berger
Sarasota Periodontal AssociatesIn the heart of Sarasota lies a dental practice that's committed to preserving your dental health—Sarasota Periodontal Associates. Our clinic specializes in a wide range of periodontal services such as Scaling & Root Planing, Recession Treatment/Gum Grafting, and Bone Grafting. If you're seeking a periodontist in Sarasota, look no further than our knowledgeable team who are well-versed in treating various periodontal conditions with expertise and compassion. For those on the lookout for dental implants sarasota, our practice offers superior implant solutions tailored to meet individual needs and restore smiles with confidence. We prioritize patient comfort by providing Mild & Moderate Sedation options during procedures like Laser Surgery and Cosmetic Periodontal Surgery. Dental health is our passion at Sarasota Periodontal Associates, where we work diligently to provide exceptional care as a trusted dentist in Sarasota for all your periodontal needs.
Call Us:
941-907-7310
view this profile




