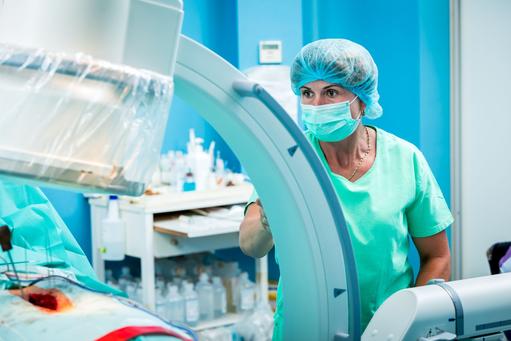
- Over the past 2 decades minimally invasive surgery and computer assisted navigation (CAN) systems have significantly changed spine surgery
- Minimally invasive spine surgery (MISS) has become a significant subspeciality accounting for ~50% of all spine surgeries undertaken in the US
- Together MISS and CAN systems promise enhanced precision, improved outcomes, and lower costs
- CAN systems provide surgeons with improved visibility of the operating site, but emit hazardous radiation that can cause cancer
- Spine surgery appears to be winning the challenge to increase the development of minimally invasive surgery while decreasing harmful radiation in the operating room
- MISS is positioned to grow and increase its market share but faces some headwinds
- Low back pain and the global spine industry -
Minimally invasive spine surgery and computer assisted navigation systems
Minimally invasive spine surgery (MISS) requires only a small incision and uses specialized instruments and techniques that minimize cutting and results in minimal damage of body tissue. The technique serves the increasing prevalence of degenerative spinal disorders, attributed to sedentary lifestyles of aging populations, which have helped to drive the growth of a global spinal implants and devices market. In addition to the increased availability of biologics and customizable implants and the refinement of operative techniques, the development of MISS has been supported by advances in imaging and navigation technologies that make surgical targets virtual on a monitor to improve the accuracy and precision of surgical interventions. Today, there is a growing body of research demonstrating MISS’s advantages over the traditional open approach. However, computer assisted navigation (CAN) systems tend to emit harmful ionizing radiation that can cause cancer. Reducing radiation in the OR while improving the quality of image guidance is expected to fuel further growth of MISS.
In this Commentary
This Commentary focuses on minimally invasive spine surgery and computer assisted navigation systems. Two technologies, which have changed the landscape of modern spine surgery and offer potential benefits for both patients and surgeons. Has MISS reached its market saturation? If not, what will affect the speed and extent of its further adoption?
Minimally invasive and open spine surgery
Over the past 2 decades, MISS has become a significant subspeciality and currently accounts for ~50% of all spine surgeries undertaken in the US. It is positioned to increase its influence over the next decade but faces some headwinds.
As a general principle, it is preferable to intrude as little as possible when carrying out a surgical procedure to minimise damage to surrounding tissue and to speed up recovery time. Many spine procedures that once required invasive operations (open surgery) have been replaced with MISS techniques.
Open spine surgery typically involves relatively long incisions down the back to give the surgeon the best view of, and access to, the anatomy. During such procedures, it is sometimes necessary to cut through and move aside muscles and tendons to reach the affected area, which can cause damage to these tissues and prolong recovery.
In MISS the surgeon makes a small incision and then inserts a device called a tubular retractor, a stiff, tube-shaped tool that creates a tunnel to the problem area of the spine by gently pushing aside the muscle and soft tissue around the affected area. The surgeon can then put small tools through the tunnel to work on the spine and use a special microscope to view real-time X-ray images of the spine. This approach results in less damage to the muscles and soft tissues that surround the spine, which leads to a more expedited recovery.
MISS has gained popularity both with patients and clinicians and has become increasingly feasible for the management of a range of spinal disorders. Progress has been made in the development of a direct lateral approach [from the side] as well as improvements of tubular retractors. Common spine surgery treatments available through minimally invasive methods include degenerative disc disorders, herniated discs, lumbar spinal stenosis, spinal deformities such as scoliosis, spinal infections, spinal instability including spondylolisthesis, vertebral compression fractures, and spinal tumours. In 2020, MISS procedures accounted for ~50% of all spine surgeries performed in the US, which had increased from ~16% in 2012.
According to David Bell, a consultant neurosurgeon at King’s College Hospital, London, who specialises in complex spine surgery, MISS significantly improves the patient experience by, “reducing the size of the incision and the amount of tissue manipulation . . . It also minimises post-operative discomfort, cuts infection rates, lessens blood loss and reduces a patient’s recuperation time”. See video below.

The evidence
There is a growing body of research to support the benefits of MISS, which include: (i) reduced trauma to muscles and soft tissue, (ii) better cosmetic results from smaller incisions, (iii) less blood loss, (iv) reduced risk of infection, (v) faster recovery time and less rehabilitation, (vi) diminished reliance on pain medications, and (vii) reduced hospital stays. A further perceived benefit is the increasing range of MISS undertaken in outpatient settings. Such benefits are likely to fuel the refinement of surgical techniques based on patient outcomes, and lead to the growth of MISS.
However, not all studies are so positive about the benefits of MISS. A 2017 review of 17 randomized controlled trials, which compared MISS against open procedures for three common disorders, concluded that, “the evidence do not support MISS over open surgery for cervical or lumbar disc herniation”. The study suggests that there were some advantages for transforaminal lumbar interbody fusion (TLIF), [a procedure that melds the front and back sections of the spine through a posterior approach], but “at the cost of higher revision rates, higher readmission rates and more than twice the amount of intraoperative fluoroscopy”. [an imaging technique employed to improve intraoperative visualization of the operating field, which emits hazardous radiation]. The study concludes that, “Regardless of patient indication, MISS exposes the surgeon to significantly more radiation”.
Two papers published in the January 2020 edition of the Journal of Spine Surgery report on a global survey of 430 surgeons to assess the extent of MISS and the training surgeons receive. The response rate was significant at 67%. 33% of respondents were neurosurgeons, 55% orthopaedic surgeons and 12% were surgeons with other postgraduate training. One research paper concludes that, “endoscopic spinal surgery is now the most commonly performed MISS technique”, and the other suggests that, “very few MISS surgeons are fellowship trained but attend workshops and various meetings suggesting that many of them are self-thought. Orthopaedic surgeons were more likely to implement endoscopic spinal surgery into the routine clinical practice”.
|