Tag
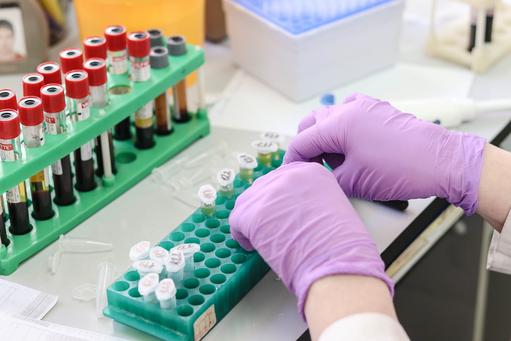
- Bioengineers throughout the world are competing to achieve the Holy Grail: an affordable, point-of-care blood test - liquid biopsy - that detects cancer before any symptoms present
- Success in achieving this will save millions of lives, substantially reduce healthcare costs and make investors, researchers and organisations billions
- Despite significant advances no one has yet achieved the Holy Grail and there remains a substantial gap between researchers’ aspirations and reality
- How close are we?
Alfattani was presenting research findings of a small study at the National Cancer Research Institute’s (NCRI) conference in Glasgow, Scotland, in November 2019, which is an international forum for showcasing cancer advances.
A September 2019 HealthPad Commentary described another early detection test for breast cancer called CanRisk, which has been developed by researchers from Cambridge University’s Centre for Cancer Genetic Epidemiology and has the potential to identify women with different levels of risk of breast cancer.
Alfattani and bioengineers from the universities of Nottingham and Cambridge are players in a vast and rapidly evolving international army of researchers engaged in an intensely competitive global race to develop an affordable, point-of-care, early detection test (EDT) for cancer based upon a liquid biopsy and next generation sequencing technologies. The Holy Grail is for such a test to detect cancer cells in an asymptomatic patient, locate the tissue of origin and give that person an early diagnosis when treatment is more likely to be successful; and to do all this with 100% accuracy.
Although Alfattani’s research study is modest, her findings are potentially clinically relevant because they are on the Holy Grail therapeutic pathway, and her preliminary findings suggest that a simple, cheap and easy-to-use blood test - liquid biopsy - could detect breast cancer five years before any symptoms present. If demonstrated to be exquisitely accurate, safe and efficient by a larger study, which already is underway at Nottingham University’s CEAC, Alfattani’s research could be a key to saving thousands of lives and substantial amounts of money.
Gold standard breast cancer screening
In this Commentary
Partial epidemiology of breast cancer
Alfattani’s research
The Nottingham researchers took blood samples from 90 breast cancer patients at the time they were diagnosed with the disease and matched them with samples taken from 90 patients without breast cancer (the control group). Researchers employed technology (protein microarray), which allowed them to screen the blood samples for the presence of autoantibodies against 40 TAAs associated with breast cancer and also 27 TAAs not known to be linked with the disease. The accuracy of the test improved in the panels that contained more TAAs.
Findings
A panel of five TAAs correctly detected breast cancer in 29% of the samples from the cancer patients and correctly identified 84% of the control group as being cancer-free. A panel of seven TAAs was able to detect disease in 35% of cases with breast cancer and rule out 79% of patients in the control group. The most successful technique was a panel of nine antigens, which correctly identified the disease in 37% of cancer samples and no cancer in 79% of the controls. “The results of our study showed that breast cancer does induce autoantibodies against panels of specific tumour-associated antigens. . . . . The results are encouraging and indicate that it is possible to detect a signal for early breast cancer. Once we have improved the accuracy of the test, then it opens the possibility of using a simple blood test to improve early detection of the disease”, said Alfattani.
David Crosby, head of early detection at the Cancer Research UK charity, said, “Diagnosing cancer at the earliest stages before it grows or spreads gives patients the best chance that their treatment will be successful. So, the potential to detect markers in the blood before other signs appear is promising”.
|
|
|
|

Neil McHugh
Consultant Rheumatologist, Royal National Hospital for Rheumatic Diseases (RNHRD), Bath, UK; Professor of Pharmacoepidemiology, University of Bath, UKDirectory:
Expertise:
Professor Neil McHugh graduated from Otago University Medical School, Dunedin, New Zealand, and completed physician training before specialising in the subspecialty of rheumatology. He has undertaken research fellowships at the Walter and Eliza Hall in Melbourne, Australia (1985), Yale University Medical School, USA (1990–1991), and the National Heart and Lung Institute, UK (2002–2004).
Professor McHugh has been a Consultant Rheumatologist at the RNHRD since 1991, and Professor of Pharmacoepidemiology at the University of Bath since 2013. His current interests include the serology of idiopathic inflammatory myositis, scleroderma, lupus and juvenile idiopathic arthritis. His laboratory group offers specialist autoimmune serology at the University of Bath, leads on a major European study of adult and juvenile idiopathic myositis, and has identified a number of novel diagnostic autoantibody biomarkers.
Professor McHugh also leads a programme of work related to psoriatic disease, including a National Institute for Health Research programme grant related to the early detection and optimisation of outcome measures in psoriatic arthritis. He has led on successive British Society for Rheumatology guidelines for biologics in psoriatic arthritis, and has been involved in National Institute for Health and Care Excellence and other international guidelines for psoriatic disease.
view this profile





