Publications
- All
-
Following
You must log in
before getting
tailored content. -
Interests
You must log in
before getting
tailored content. - Most liked
- Most viewed
Directory:
Tags:

- Prime editing devised by researchers at the Broad Institute led by David Liu is a significant advance of the original CRISPR gene editing tool discovered in 2012
- CRISPR can cut and edit your DNA to correct defects inside your body’s cells to prevent and heal a range of incurable diseases and has revolutionized biomedicine
- The original CRISPR is fraught with inaccuracies referred to as off target effects
- Prime editing substantially reduces CRISPR’s off target effects and has the potential to correct up to 89% of known disease-causing genetic variations
- CRISPR also has the capacity to edit genes in an embryo in such a way that the change is heritable
- In 2018 Chinese researcher He Jiankui “created” the world’s first CRISPR babies
- This triggered international criticism from scientists and bioethicists
- A principal concern is that CRISPR is easy-to-use, cheap, regularly used in thousands of laboratories throughout the world and there is no internationally agreed and enforceable regulatory framework for its use
In 2012 the world of biomedicine changed when a revolutionary gene editing technology known as CRISPR-Cas9 (an acronym for Clustered Regularly Interspaced Short Palindromic Repeats) was discovered. The technology harnesses your body’s naturally occurring immune system that bacteria use to fight-off viruses and has the potential to forever change the fundamental nature of humanity. Since its discovery CRISPR has been developing at lightning speed primarily because it is simple and affordable and today is used in thousands of laboratories throughout the world.
We also review a case where an ambitious scientist “created” the first CRISPR babies. This immediately triggered international criticism and a call for tighter regulatory control of the technology. Scientists and bioethicists are concerned that CRISPR can easily be used to create heritable DNA changes, which ultimately could lead to ‘designer babies’.
These two accounts of CRISPR might seem “opposites” and not sit well together in a single Commentary. Notwithstanding, what prompted putting them together was John Travis, the News Managing Editor of the well-known scientific journal Science, who soon after CRISPR’s discovery in 2012 said, “For better or worse we all now live in CRISPR’s world”.
CRISPR and your DNA
CRISPR is different to traditional gene therapy, which uses viruses to insert new genes into cells to try and treat diseases and has caused some safety challenges. CRISPR, which avoids the use of viruses, was conceived in 2007 when a yogurt company identified an unexpected defence mechanism that its bacteria used to fight off viruses. Subsequent research made a surprising observation that bacteria could remember viruses. CRISPR has been likened to a pair of microscopic scissors that can cut and edit your DNA to correct defects inside your body’s cells to prevent and heal a range of intractable diseases. The standard picture of DNA is a double helix, which looks similar to a ladder that has been twisted. The steps in this twisted ladder are DNA base pairs. The fundamental building blocks of DNA are the four bases adenine (A), cytosine (C), guanine (G) and thymine (T). They are commonly known by their respective letters, A, C, G and T. Three billion of these letters form the complete manual for building and maintaining your body, but tiny errors can cause disease. For example, a mutation that turned one specific A into a T results in the most common form of sickle cell disease.
The original CRISPR
Subsequently however, the paper was retracted, and an error correction was posted on a scientific website. Contrary to their original findings, the authors of the Nature Biotechnology paper restated that the CRISPR-Cas9 gene editing approach, "can precisely edit the genome at the organismal level and may not introduce numerous, unintended, off-target mutations".
Notwithstanding, researchers remained concerned about CRISPR’s off target effects and several devised a technique, referred to as base editing, to reduce these. Base editing is described in three research papers published in 2017: one in the November edition of the journal ‘Protein and Cell’, another in the October edition of ‘Science’ the and a third by researchers from the Broad Institute, in the October edition of the journal ‘Nature’. Base editing takes the original CRISPR-Cas9 and fuses it to proteins that can make four precise DNA changes: it can change the letters C-to-T, T-to-C, A-to-G and G-to-A. The technique genetically transforms base pairs at a target position in the genome of living cells with more than 50% efficiency and virtually no detectable off-target effects. Despite its success, there remained other types of point mutations that scientists wanted to target for diseases.
|
|
|
|
Directory:
Tags:

- Diabetic foot ulcers (DFUs) are a result of diabetes complications and can lead to amputations and death
- Scientists and clinicians struggle to reduce the vast and escalating burden of DFUs
- In wealthy countries like the UK there are specialist multidisciplinary diabetic foot clinics
- New and innovative therapies are beginning to emerge, which accelerates the rate of complete wound closure for DFUs
- Notwithstanding new products coming to market, the best therapy is prevention
This Commentary discusses diabetic foot ulcers (DFUs) within the context of chronic wounds. Although chronic wounds tend to be an overlooked area of medicine and do not feature prominently in the popular media; NHS England, spends £5bn a year treating 2m patients with chronic wounds. The incidence rates of people affected with wounds are rising fast and some experts suggest that nearly 60% of all wounds become chronic. According to Una Adderley, a wound expert and Director of NHS England’s National Wound Care Strategy Programme, therapy in England for chronic wounds is patchy and suboptimal, “leading to non-healing or delayed healing (which) increases the number of people living with chronic wounds. Too many people are receiving care for which there is little evidence that it works and too few are receiving care for which there is strong evidence that it works”.
According to a 2019 report by the consulting firm MarketsandMarkets the global wound care market in 2019 is estimated to be US$20bn and projected to reach US$25bn by 2024. Market drivers include the vast and fast-growing incidence rates of hard-to-heal chronic wounds, a large proportion of which are associated with diabetes, increasing R&D spending, technological developments, the growing use of regenerative medicine in wound care, recent advances in molecular data that have contributed to genome sequencing, and the increasing use of AI in the management of wound care solutions. The chronic wound care markets of North America and Europe are expected to grow at a CAGR of ~4.5% for the next 5 years, but the highest CAGR is expected in Asia where the vast pool of patients is increasing significantly, and favourable reimbursement policies are expected to persist in the region for the next decade.
When accompanied by an underlying condition such as diabetes, chronic wounds in the form of DFUs, are challenging to heal and have a deleterious effect on your quality of life: you experience pain, suffering, disfigurement, anxiety impaired mobility, malodour and social isolation. Because the prevalence of diabetes is increasing worldwide, DFUs have become a large, severe and growing public health issue as described in two research papers published in 2019.
One, published in the May 2019 edition of Diabetic Medicine, reports findings of an 18 year study of DFUs, and suggests that although current therapies in the UK result in better than previously reported survival in persons < 65 years (10 year survival is 85%), treatments fail to, “reduce recurrent incidence (of DFUs and) cumulative prevalence of all ulcers continues to increase”; from 20.7 to 33.1 per 1,000 persons between 2003 to 2017. The second paper, published in the January-March 2019 edition of the International Journal of Applied Basic Research, report sfindings of a prospective Indian study of 63 patients >18 with DFUs and shows the increase in the severity of DFUs and the consequent increase in the rate of hospital readmissions, amputations and mortality.
Diabetes is a chronic disease that occurs either when your pancreas does not produce enough insulin or when your body cannot effectively use the insulin it produces. Insulin is a hormone that regulates your blood sugar level. High blood sugar levels (hyperglycaemia) is a common effect of uncontrolled diabetes and can lead to serious complications, which include blindness, kidney failure, heart attacks, stroke, diabetic foot ulcers (DFUs), and lower limb amputations. According to the World Health Organization, the global prevalence of diabetes among people >18 has risen from 4.7% in 1980 to 8.5% in 2014. Today, some 422m people worldwide have diabetes, which has increased from 108m in 1980. There is expected to be some 642m people >18 living with diabetes by 2040.
If you have diabetes you are prone to ulcers because your increased blood sugar levels create thick, sticky blood, which can lead to peripheral artery disease (PAD), neuropathy (a loss of sensation due to nerve damage), and/or problems with circulation due to damage to your small blood vessels, which reduce your body’s ability to heal injuries.
Signs and symptoms of DFUs include numbness in your toes and a loss of feeling in your feet, painful tingling sensations, blisters, minor abrasions and cuts without pain that do not heal, skin discoloration and temperature changes With a loss of sensation, a minor injury to your foot can go unnoticed and untreated, and quickly lead to an ulcer. If you are living with diabetes, ulceration is an ongoing challenge. Only about 66% of DFUs eventually heal without surgery. If you have had a foot ulcer you are at increased risk of further ulceration. Studies suggest that around 25% of people living with diabetes who become ulcer-free have developed new ulcers within 3 months, and 34% to 41% within 12 months. Some foot ulcers are painful, and treatment often requires that you spend a significant amount of time visiting clinics to frequently change your wound dressings. The poor prognosis of DFUs is often attributed to other complications of diabetes such as peripheral neuropathy, peripheral vascular disease and persistent hyperglycaemia. Managing diabetic foot ulcers is a major challenge for healthcare systems globally and the main cause of more than half of nontraumatic lower limb amputations: every 30 seconds in the world, a lower limb is amputated due to diabetes. Amputations have life-altering repercussions for patients and represent a significant burden for the healthcare industry as a whole. Between 0.03% and 1.5% of people with DFUs require an amputation and most amputations start with ulcers.
For major amputations, the prognosis is poor because your other limb is at risk. Research suggests that only around 50% of patients survive for two years after major diabetes related amputations. The one-year mortality rate has been estimated at 32.7% after major amputation and 18.3% after minor amputation if you have diabetes. Five-year cumulative mortality for patients with diabetes undergoing a first major amputation has been estimated at 68% to 78.7%. Thus, if you have diabetes and a DFU you have almost a 50% chance of being dead within five years, which is significantly higher than for people with either breast (18%) or prostate (8%) cancers.
In the UK some 70,000 to 90,000 people living with diabetes have DFUs at any one time. If you have diabetes you are about 23 times more likely to experience an amputation than someone without diabetes. In England, diabetes leads to more than 9,000 lower limb amputations each year. Each week in England some 169 people undergo an amputation procedure as a result of diabetes. Analysis by the charity Diabetes UK found that between 2014 and 2017, 26,378 people had lower limb amputations linked to diabetes, which represented a 19% rise from 2010 to 2013. Diabetes affects almost 3.7m people in the UK. In 2017 NHS England launched a special transformation fund aimed at improving patients with diabetes access to specialist multidiscipline foot care clinics to help avoid amputations.
In the video below Hisham Rashid, Consultant Vascular Surgeon at King’s College Hospital, London, describes a DFU and explains why they benefit from specialist multidisciplinary treatment centres. “DFUs have similar features to other ulcers, and often present in the toes and heal areas of the foot with the loss of skin and an exposed base with infection and necrosis. The significant difference is that a DFU usually comes with multiple pathologies, which, in addition to infection, include neuropathy and peripheral vascular disease. DFUs do not heal quickly and often require vascular surgeons working closely with radiologists, orthopaedic surgeons to correct any deformity and a microbiology unit to manage infection,” says Rashid.
Rashid also explains that different therapies are used to heal DFUs. “If the patient has peripheral artery disease (ischaemia) then this has to be treated first with an angioplasty or a bypass or both to improve blood circulation into the foot. Once this is achieved, the ulcer is debrided and dressed. There are different dressings, which include negative pressure dressing, which sucks the blood into the tissues and thereby promotes healing. Sometimes skin graphs are necessary to get the tissue to heal faster. This can be done as a day surgery using local anaesthetic,” says Rashid.
Prevention of DFUs
Given the severity of DFUs and their vast and rapidly increasing burden on individuals with diabetes and healthcare systems, increasing attention is being devoted to prevention, which involves adequate glycaemic control and modification of risk factors. While education is an obligation of healthcare professionals, it is crucial that people living with diabetes themselves increase their awareness and understanding of the condition and integrate regular feet examination and care into their daily lives. In the video below, Roni Sharvanu Saha, Consultant in Acute Medicine, Diabetes and Endocrinology, St George’s Hospital, London, suggests that, “We’re getting better at understanding why DFUs occur, and better at examining peoples’ feet. In England, if you have diabetes you are entitled to a clinical examination of your feet at least twice a year. Checks include whether you have any minor abrasions, or whether you can distinguish hot and cold water with your feet, and signs that you might have problems with your circulation and nervous system. Ensuring that people living with diabetes receive regular checks means that if you have reduced or poor circulation, you’re referred to the correct specialty team in order to protect you from developing DFUs. Prevention is better that cure. If we can get better at examining feet, outcomes will improve. If diabetes is not controlled complications will occur”.
With the well-being of millions of people living with diabetes at stake, there is a pressing need for therapies that bring DFUs to closure as quickly as possible. The current standard of care (SOC) regimen for DFUs involves maintaining a moist wound environment, debriding nonviable tissue, relieving pressure with an offloading boot and preventing or managing wound infection. Even with a good SOC, DFUs are notoriously slow to close, creating a demand for new and innovative medicines and techniques to enhance closure. Increasingly, there are advanced therapies to facilitate healing DFUs when traditional approaches fail.
An example of a relatively new product to help close DFUs is human amniotic membrane. Amniotic membrane has been used for wound healing purposes since the early 20th century, but it represents a relatively recent and promising advanced therapy to accelerate healing in DFUs. Amniotic membrane is derived from the human placental sac that supports the foetus by forming the inner lining of the amniotic cavity. Functions of amniotic membrane include the exchange of water-soluble molecules and the production of cytokines and growth factors to facilitate the development of the foetes. The anatomic makeup of amniotic membrane dictates its functionality, and a significant characteristic is its ability to produce a wide variety of regenerative growth factors that facilitate foetal development. These growth factors, in combination with various other cytokines, have substantial potential benefits in wound healing, which include creating a structural scaffold for tissue proliferation, modulating the immune response, reducing inflammation, stimulating angiogenesis and facilitating tissue re-modelling.
Two small but significant prospective cohort studies on the effectiveness of human amniotic tissue to treat DFUs were reported in the journal Wounds. One in the March 2016 edition and another in the November 2017 edition. The first is a prospective, randomized, multicentre, controlled study and the second a retrospective cohort study of 20 patients. In both studies amniotic membrane is used in combination with SOC, including debridement, well-controlled offloading, management of bacterial burden, and adequate perfusion.
Both studies suggested that the use of amniotic membrane is more likely to: (i) lead to complete wound closure, (ii) accelerate the rate of wound closure, and (iii) present no additional safety risks when compared to SOC alone in the treatment of DFUs. The first study demonstrated a statistically significant advantage of an amniotic membrane as compared to SOC in facilitating closure of chronic DFUs. 45% of participants achieved complete wound closure, while 0% of SOC participants alone achieved complete wound closure within 6 weeks. Further, there appears to be no increased rate of adverse events associated with the use of amniotic membrane in these wounds. The second study was a retrospective cohort study using a human amniotic membrane on 20 patients presenting with DFUs and venous leg ulcers. Patients underwent a 2-week ‘run-in’ period with good SOC; and if upon their return the ulcer had closed ≥ 30% in area, the subject was excluded from participation in the study. All wounds were effectively closed in approximately 10 weeks, DFUs in 12 weeks and venous leg ulcers in 9 weeks, and no adverse events were noted, suggesting that the therapy using human amniotic membrane is safe.
The most significant limitation of both studies is their small sample size, which decreases the generalizability of their findings. Notwithstanding, the studies suggest that amniotic tissue products are efficacious options for DFUs when used in conjunction with the current SOC, which includes aggressive sharp debridement, adequate offloading and the application of sterile dressings. Further, amniotic membrane, like most biologic tissue products, requires significant processing and therefore its cost is relatively high: on average between US$500 to US$1,000 per application. Notwithstanding, these costs are significantly less than the average annual therapy cost of US$28,000 per patient for SOC for a DFU. And therefore, using amniotic tissue in the therapy for DFUs could result in significant savings for healthcare systems. Tissue storage as well as the time and skill required to apply amniotic membranes also represent challenges inherent to these products.
Millions of people are living with diabetes, which, if not managed appropriately can lead to life-changing complications. A DFU is one such complication, which often starts with a minor abrasion on your ankle or toe that you do not feel and therefore tend not to perceive to be important, until that is, it quickly escalates into a chronic wound that does not heal and eventually leads to a lower limb amputation. In most wealthy nations, health providers are aware of the dangers of DFUs and have set up multi-disciplinary diabetic foot clinics to treat and manage the condition. However, access to such clinics is patchy and the prevalence of DFUs continues to increase, and the eye-watering costs of treating and managing DFUs continue to escalate. In recent years, the therapy for DFUs has been improved by technological advances. We describe one of these: the use of amniotic tissue in conjunction with standard of care protocols. Recent research findings suggest that the use of amniotic tissue holds out the possibility not only of significant therapeutic benefits, but also of substantial cost savings for healthcare systems. Notwithstanding, perhaps the most efficacious therapy for DFUs is prevention. This means investing in effective education and awareness programs, good glycaemic control and appropriate footwear; encouraging people living with diabetes to participate in regular foot examinations and screening for peripheral neuropathy and peripheral arterial disease, and insisting that early telltale signs of foot wounds, no matter how minor, should be immediately referred to a specialist clinic.
Directory:
Tags:
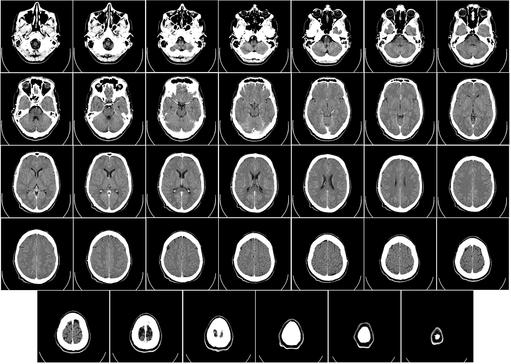
- Hydrocephalus is a chronic condition that occurs when excess cerebrospinal fluid (CSF) collects in your brain’s ventricles and increases pressure inside your head
- Failure to treat the condition can lead to morbidity and death
- First line therapy is the surgical insertion of a ventriculoperitoneal shunt (VPS) to restore your CSF circulation
- A significant risk with the procedure is infection
- To reduce infection manufacturers’ impregnate standard shunts with either silver or antibiotics and market the impregnated shunts at higher prices
- Which VPS (standard, silver or antibiotic) provides patients with the most protection from infection?
- Which VPS is most cost effective for healthcare systems?
“It” is Hydrocephalus; a chronic condition that occurs when excess cerebrospinal fluid (CSF) collects in your brain’s ventricles, (fluid-filled areas). CSF disperses from your ventricles around your brain and spinal cord. Too much CSF may result in an accumulation of fluid, which can cause the pressure inside of your head to increase. In a child, this causes the bones of an immature skull to expand and separate to a larger-than-normal appearance.
There are no medical therapies to effectively treat hydrocephalus. The only viable treatment is surgical. The gold standard therapy is the insertion of a ventriculoperitoneal shunt (VPS), which is a common surgical procedure to restore your CSF circulation, regulate its flow and allow you to have a normal daily life. Notwithstanding, a significant challenge is infection at the site of the surgical wound, the shunt or in the cerebrospinal fluid itself (meningitis). This effects about 15% of hydrocephalus patients and may result in further surgeries, extended hospital stays, a reduction in your quality of life and a significant hike in healthcare costs.
To reduce potential infection manufacturers’ impregnate standard shunts with either silver (silver has benefits in reducing or preventing infection) or antibiotics and market the impregnated shunts at higher prices.
There are two principal classifications for hydrocephalus: (i) communicating and (ii) non-communicating hydrocephali. Both can be subdivided into congenital (present at birth) and acquired (occurs following birth). Communicating hydrocephalus can also be subdivided into normal pressure hydrocephalus (NPH) and hydrocephalus ex-vacuo, which occurs when there is damage to your brain caused by stroke or injury. It is generally understood that congenital hydrocephalus can be caused by genetic defects, which can be passed from one or both parents to a child, but the direct hereditary links are still being investigated. Notwithstanding, experts have found a connection between a rare genetic disorder called L1 syndrome and hydrocephalus. L1 syndrome is a group of conditions that mainly affects the nervous system and occurs almost exclusively in males.
Most babies born with hydrocephalus or who develop hydrocephalus as infants will have a normal lifespan, and approximately 40 to 50% will have normal intelligence. Seizure disorders have been diagnosed in about 10% of children with hydrocephalus and the mortality rate for infants is approximately 5%.
In the video below Sanj Bassi, a Consultant Neurosurgeon at King’s College Hospital, London and a member of the London Neurosurgery Partnership, describes hydrocephalus:

Causes
Signs and symptoms
Diagnosing hydrocephalus
In the video below Bassi describes how hydrocephalus is diagnosed:
A shunt consists of two thin, long flexible hollow tubes, called catheters, with a valve that keeps fluid from your brain flowing in the right direction and at the proper rate and thereby reduces brain pressure to a safe level. To install a shunt a surgeon will make a small insertion behind your ear and also drill a small borehole in your scull. One catheter is then threaded into one of your brain’s ventricles through the hole in your scull, and the other is inserted behind your ear and threaded subcutaneously down to your chest and into your abdomen where excess CSF can drain safely, and your body can reabsorb it. Your surgeon may attach a tiny pump to both catheters and place it under the skin behind your ear. The pump will automatically activate to remove fluid when the pressure in your skull increases. Shunts can be programmable (externally adjustable by a magnetic device) to activate when the fluid increases to a certain volume, or non-programmable. Most surgeons tend to choose a programmable model, despite the fact that in clinical studies both types perform comparably.
To determine the relative clinical benefits and cost-effectiveness of the three different ventriculoperitoneal shunts (standard, silver or antibiotic) following their de novo insertions, the UK’s National Institute for Health Research funded a large prospective multi-centre randomised controlled clinical study - The British Antibiotic and Silver Impregnated Catheters for Ventriculoperitoneal Shunts Study - (BASICS). Findings were published in the September 2019 edition of The Lancet. These concluded that shunts impregnated with antibiotics significantly reduce the risk of infection and also healthcare costs compared to both standard shunts and those impregnated with silver. Conor Mallucci, Consultant Paediatric Neurosurgeon at Alder Hey Children’s Hospital, Liverpool, UK, and lead author of the study, suggests that shunts impregnated with antibiotics should be, “the first choice for patients with hydrocephalus undergoing insertion of their first ventriculoperitoneal shunt”.
All shunts used in the study were CE marked medical devices intended for the condition. Participants were randomly assigned to three groups: one group of 536 received a standard shunt, another of 531 received a silver impregnated shunt, and a third group of 538 received an antibiotic impregnated shunt. The minimum patient follow-up period was six months and the maximum two years. Six per cent of evaluable patients in both the standard and silver groups presented with infections and required a shunt revision. This compared to only 2% in the antibiotic impregnated shunt group that became infected and needed revising. The difference is significant.
The Study’s economic findings
The research has a further significance because, despite the high medical costs of treating hydrocephalus, the annual spend on hydrocephalus research is relatively low. For example, the US National Institutes of Health (NIH) invests less than US$8m per year in hydrocephalus research. This means that there is a dearth of clinical studies associated with the condition and no long-term follow-up research over the lifetime of patients.
Although BASICS is a significant study it should be mentioned that it is restricted by the relatively low proportion of patient-reported outcomes: 32, 31 and 12 reported infections after insertion of the standard, silver and antibiotic VPS’s respectively.
Takeaways
Directory:
Tags:

- CanRisk is a new online gene-based health-risk evaluation algorithm for detecting breast cancer
- It identifies people with different levels of risk of breast cancer, not just those at high risk
- As the infotech and biotech revolutions merge expect authority in medicine to be transferred to algorithms
- CanRisk has the potential to provide a cheap, rapid, non-invasive, highly sensitive and accurate diagnosis before symptoms present
- Breast cancer is the most common cancer in women worldwide and is the 5th most common cause of death from cancer in women
- Currently mammography screening, which has a sensitivity between 72% and 87%, is the gold standard for preventing and controlling breast cancer
- For every death from breast cancer that is prevented by screening, it is estimated there will be three false-positive cases that are detected and treated unnecessarily
- Lack of resources do not support breast cancer screening in many regions of the world where the incidence rates of the disease are rapidly increasing
- In the near-term expect interest in the CanRisk algorithm to increase
Although over the past two decades there have been significant improvements in the detection and treatment of breast cancer, the disease remains the most common cancer in women worldwide, with some 1.7m new cases diagnosed each year, which account for about 25% of all cancers in women and it is the fifth most common cause of death from cancer in women, with over 0.52m deaths each year.
When fully developed and approved, CanRisk will be well positioned to provide a cheap, rapid, non-invasive, highly sensitive and accurate diagnostic test to detect breast cancer early in people with diverse levels of risk. This might be expected to provide an alternative to the current gold standard population-based mammography screening and assist in making a significant dent in the vast and escalating global burden of the disease.
|
|
|
|
Directory:
Tags:
- Over the past decade MedTech valuations have outperformed the market without changing its business model
- The healthcare ecosystem is rapidly changing and MedTech is facing significant headwinds which require change
- MedTech’s future growth and value will be derived from data and smart analytics rather than manufacturing
- MedTech leaders will be required to leverage both physical and digital assets
According to a 2018 report by the consulting firm Ernst & Young,“Stagnant R&D investment, low revenue growth and slow adoption of digital and data technologies suggest that entrenched MedTech companies are overly focused on short-term growth, even as the threat of large tech conglomerates entering the space grows larger, which, in addition to the changing global healthcare ecosystem, threatens future revenue growth".
The medical device industry
Concern # 1: Reduced growth rates
Population growth and aging
Concern # 2: Stagnate R&D spend and share buybacks
To the extent that share buybacks extract, rather than create value why are they popular? One suggestion is that because share incentive plans represent a significant portion of executive compensation, share buybacks make it easier for executives to meet earning-per-share (eps) targets by reducing the number of shares, in the 1970s, share buybacks were effectively banned in the US amid concerns that executives might use them to manipulate share prices. However, in 1982 the US Securities and Exchange Commission (SEC) lightened its definition of stock manipulation, and share buybacks became popular again.
|
|
|
|
Directory:
Tags:
- Experts have called for the worldwide eradication of cervical cancer, but this is not likely to happen for a long time
- Significant progress has been made to eliminate cervical cancer in developed countries
- The overwhelming burden of cervical cancer falls disproportionately on women in low- to middle-income countries (LMIC)
- LMIC have relatively low levels of awareness of cervical cancer, patchy prevent programs and limited treatment options
- Over 80% of cervical cancer cases and deaths occur in LMIC
- Cervical cancer is the fourth most common cancer in women worldwide
- In 2018 there were an estimated 680,000 new cases and 311,000 deaths from the disease worldwide
- Cervical cancer is caused by sexually acquired infection from high-risk strains of the human papilloma virus (HPV)
- The majority of women will be infected with HPV at some point in their life
- HPV also causes genital warts and cancers of the head and neck and is also linked to cancers of the anus, vulva, vagina, penis and oropharynx
- HPV vaccines protect against 70% of cervical cancers and about 90% of genital warts
- Regular screening is also recommended to reduce the incidence of cervical cancer
Cervical cancer is a killer disease, which only affects women. It affects women of all ages from schoolgirls to grandmothers, but it is significantly more prevalent between the ages of 30 and 45.
The cervix, also known as the neck of the womb, connects a woman's womb and her vagina.
Cervical cancer is the fourth most common cancer in women worldwide and second for women between 15 and 44. In 2018 there were an estimated 680,000 new cases and 311,000 deaths from the disease worldwide. The overwhelming majority of cases are caused by two specific strains of the human papilloma virus (HPV). HPV infection and early cervical cancer typically do not present noticeable symptoms, and cervical cancer may take 20 years or longer to develop after an HPV infection. The overwhelming global burden of the disease falls disproportionately on women in low- to middle income countries (LMIC). There is a significant and growing gap in the incidence and mortality rates of cervical cancer between developed nations and LMIC. Despite international efforts, it seems unlikely that this gap will be narrowed in the medium term.
In this Commentary
About 79m Americans are currently infected with HPV, with roughly 14m people becoming newly infected in the US each year. In the UK, HPV is present in one in three people and 90% of individuals will come into contact with some form of the virus in their lifetime. About 80% of sexually active people are infected with HPV at some point in their lives, but most people never know they have the virus. Whitfield Growdon, a surgical oncologist at the Massachusetts General Hospital and professor at the Harvard University Medical School describes the HPV vaccination as, “one of the most meaningful interventions for reducing cervical cancer”; see video below.
All girls and boys aged between 11 and 12 should get the HPV vaccination. Every year in the US, over 13,000 males contract cancers caused by HPV. Catch-up HPV vaccines are recommended for girls and women through the age of 26, and for boys and men through the age of 21, if they did not get vaccinated when they were younger. HPV vaccination is also recommended for the following people, if they did not get vaccinated when they were younger: (i) young men who have sex with men through the age of 26, (ii) young adults who are transgender through the age of 26 and (iii) young adults with certain immunocompromising conditions (including HIV) through the age of 26.
The HPV DNA test determines the most likely cause of cervical cancer by looking for pieces of DNA in cervical cells and is recommended for women over 30 and not for women under 30. This is because women in their 20s tend to be more sexually active and therefore are more likely (than older women) to have an HPV infection that will go away on its own. Results of an HPV DNA test carried out on a woman in her 20s is not as significant as in and older woman and also may be confusing. The HPV DNA test can also be used in women who have slightly abnormal Pap test results to find out if they might need more testing or treatment.
A research paper about the Australian initiative published in the January 2019 edition of The Lancet Public Health concludes that, “the annual incidence of cervical cancer in Australia is likely to decrease to fewer than six new cases per 100 000 women by 2020 (range 2018–22) and to fewer than four cases per 100 000 women by 2028 (2021–35). The annual incidence of cervical cancer could decrease to one new case per 100 000 by 2066 (2054–77) if the existing HPV-based screening program continues in cohorts who are offered the nonavalent vaccine”; [a nonavalent vaccine works by stimulating an immune response against nine different antigens, such as nine different viruses or other microorganisms]. According to Suzanne Garland, Professor and Clinical Director of Microbiology and Infectious Diseases at the Royal Women’s Hospital, Melbourne, Australia, who led the research, “within 40 years the number of new cases of cervical cancer [in Australia] is projected to drop to just a few”.
The expansion of screening programs for cervical cancer in LMIC is only part of the answer to closing the gap with developed nations and eradicating cervical cancer globally. It is imperative that screening is linked to increased access to effective treatment for women with cervical cancer, particularly in its early stages when it is still curable. In LMIC there is often not only reduced access to preventive HPV vaccines and screening, but limited access to treatment and trained personnel. Notwithstanding, there is evidence to suggest that, in LMIC less-invasive and less–resource-intensive treatment options can be effective and are increasingly being made available.
According to Danielle Rodin, lead author and Radiation Oncologist at the Princess Margaret Cancer Centre, University of Toronto, Canada, "Vaccination is hugely important, but we can't neglect the millions of women who are contracting cervical cancer and dying in pain without access to treatment. These are women who have curable cancers: even advanced cervical cancer can be cured with radiotherapy. The possibility exists to make this treatment universally available". Radiation therapy makes small breaks in the DNA inside cells. This stops cancer cells from growing and dividing and causes them to die. Unlike cisplatin therapy, [an anti-cancer ("antineoplastic" or "cytotoxic") chemotherapy], which usually exposes the whole body to cancer-fighting drugs, radiation therapy is usually a local treatment.
“We know that when administered together (chemoradiation) you can give lower doses of both and get a better kill-rate on the tumour. This is now the backbone of cervical cancer therapy”, says Growdon; see video below.
Although the UAE is among the few countries to have relatively low incidence rates of cervical cancer, the disease still ranks as the third most frequent cancer among women in the UAE and the third most frequent cancer among women between 15 and 44. Estimates suggest that every year, 93 women are diagnosed with cervical cancer and 28 die from the disease in the UAE. Although Abu Dhabi is successfully leading the fight against cervical cancer and provides a roadmap for others to follow, the incidence of cervical cancer in the Middle East generally is expected to more than double by 2035 (>33,000 cases) and be responsible for more than 18,000 deaths. In some countries including Morocco and Saudi Arabia, low societal awareness and relatively low levels of screening results in about one in four women with HPV.
Directory:
Tags:

- AstraZeneca has turned traditional biopharma R&D on its head and is targeting early stage cancer
- This strategy benefits from some of AstraZeneca’s R&D endeavours
- But the strategy faces strong headwinds, which include significant technological and market challenges and substantial Competition from at least two unicorns
Baselga is AstraZeneca's new cancer research chief who has turned traditional biopharmaceutical drug development on its head by announcing AstraZeneca’s intention to target early- rather than late-stage cancer. “We need to spend our resources on those places where we can cure more people and that’s in early disease”, says Baselga, who knows that early detection can significantly improve patient survival rates and quality of life, as well as substantially reducing the cost and complexity of cancer treatment. Baselga also must know his strategy is high risk. Will it work?
In this Commentary we discuss the drivers and headwinds of AstraZeneca’s strategy to increase its R&D focus on early stage cancer. But first we briefly describe cancer, the UK’s situation with regard to the disease and explain why big pharma targets advanced cancers. Also, we provide a brief description of AstraZeneca’s recent history.
Cancer occurs when a normal cell’s DNA changes and multiplies to form a mass of abnormal cells, which we refer to as a tumour. If not controlled and managed appropriately the tumour can spread and invade other tissues and organs. In the video below Whitfield Growdon, a surgical oncologist at the Massachusetts General Hospital in Boston US, and a Professor at the Harvard University Medical School explains.
Why big pharma targets advanced cancers?
Studies in developed economies suggest that treatment costs for early-diagnosed cancer patients are two to four times less expensive than treating those diagnosed with advanced-stage cancer. Notwithstanding, there are physical, psychological, socio-economic and technical challenges to accessing early cancer diagnosis and these conspire to delay cancer detection. Thus, big pharma companies have traditionally aimed their new cancer drugs at patients with advanced forms of the disease. This provides pharma companies access to patients who are willing to try unproven therapies, which significantly helps in their clinical studies. And further, big pharma is advantaged because regulators tend to support medicines that slow tumour growth and prolong life, albeit by a few months.
Imfinzi: the only immunotherapy to demonstrate survival at three years
Findings presented at the June 2019 meeting of the American Society of Clinical Oncology (ASCO), build on a clinical study of Imfinzi reported in the September 2018 edition of The New England Journal of Medicine, and suggest that Imfinzi is the only immunotherapy to demonstrate survival at three years in unresectable stage-III NSCLC. AstraZeneca has begun a phase-3 clinical study of the PD-L1 inhibitor protein in stage II NSCLC patients.
|
|
Directory:
Tags:
- People are living longer, the prevalence of age-related degenerative disc disease is increasing and sufferers are more and more turning to spinal implant surgery as a solution
- As this significantly raises the burden on over-stretched healthcare systems, so is spine surgery increasingly becoming a key target for cost reduction within healthcare systems
- This intensifies the pressure on manufacturers to innovate and make spinal implants more cost effective
Can 3D printing and the use of new alloys reduce the high costs of producing and marketing spinal implants?
On January 8th 2019 surgeons at Joseph Spine, a specialist surgery centre based in Tampa Bay Florida, were the first in the US to implant a 3D printed interbody fusion device, which was produced by Osseus Fusion Systems. The company uses its proprietary 3D printing technology, also known as additive manufacturing, to build spinal implants from titanium material that is optimized for bone fusion and biological fixation. In August 2018, a suite of Osseus’s devices received clearance from the US Food and Drug Administration (FDA) for a range of heights and lordotic (inward spinal curvature) angles, which make them adaptable for a variety of patient anatomies. The interbody fusion devices work by being packed with biomaterials and bone grafts and inserted in between two vertebrae, where they fuse with the spine and work to prevent back pain.
3D printing
Spine surgery increasing significantly
Findings of a study published in the March 2019 edition of Spine, entitled, “Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States 2004 to 2015”, report that the rate of elective lumbar fusion surgeries in the US has increased substantially over the past decade. Such trends are indicative of most advanced industrial societies, which are changing and ageing, primarily driven by improvements in life expectancy and by a decrease in fertility. This results in people living longer, reaching older ages and having fewer children later in life. Over the next decade, these market drivers are expected to make spine surgery a key target for cost reduction within healthcare systems and this, in turn, is likely to increase pressure on manufacturers of spinal implants to make spine surgery more cost effective.
|
|
|
|
Directory:
Tags:

- Two Boston Consulting Group studies say MedTech innovation productivity is in decline
- A history of strong growth and healthy margins render MedTechs slow to change their outdated business model
- The MedTech sector is rapidly shifting from production to solutions
- The dynamics of MedTechs' customer supply chain is changing significantly and MedTech manufacturers are no longer in control
- Consolidation among buyers - hospitals and group purchasing organisations (GPO) - adds downward pressure on prices
- Independent distributors have assumed marketing, customer support and education roles
- GPOs have raised their fees and are struggling to change their model based on aggregate volume
- Digitally savvy new entrants are reinventing how healthcare providers and suppliers work together
- Amazon’s B2B Health Services is positioned to disrupt MedTechs, GPOs and distributors
- MedTech manufacturers need to enhance their digitization strategies to remain relevant
According to the BCG’s 2017 study, “Overall, innovation productivity [in the MedTech sector] is in decline. In some product categories, low-cost competitors - including those from emerging markets - have grown rapidly and taken market share from established competitors. At the same time, purchasers are becoming more insistent on real-world evidence that premium medical devices create value by improving patient outcomes and reducing the total costs of care”. The growth and spread of value-based healthcare has shifted the basis of competition beyond products, “toward more comprehensive value propositions and solutions that address the entire patient pathway”. In this environment, MedTechs have no choice but to use data to deliver improved outcomes and a better customer experience for patients, healthcare providers and payers.
|
|
|
|



























