Search results
Publications by Dr Matthew Banks
Reviews
Banks MR
Should patients expect their colonoscopy to reach the standards experienced by bowel cancer screening patients?
Frontline Gastroenterol 2012;3:122-123
Mannath J, Banks MR
Emerging technologies in Endoscopic Imaging
F1000 Med Rep. 2012;4:3. Epub 2012 Feb 1.
Kent A & Banks MR
Functional Gastrointestinal Disorders: Diarrhea
Gastroenterology Clinics of North America. Hunt (eds),
Sept 2010;39(3):495-507
Banks M.
The modern investigation and management of gastro-oesophageal reflux disease. Clinical Medicine
2009;9(6):1-5
Burleigh DE & Banks MR
Stimulation of intestinal secretion by vasoactive intestinal peptide and cholera toxin. Autonomic Neuroscience.
2007;133(1):64-75
Farthing MJG, Casburn-Jones A, Banks MR
Diarrhoea. Prescriber 2003;14 (20):48-59.
Farthing MJG, Casburn-Jones A, Banks MR.
Getting control of intestinal secretion: thoughts for 2003.
Digestive and Liver Disease 2003;35:378-385
Banks MR, Farthing MJG.
Fluid and electrolyte absorption. Current Opinion in Gastroenterology 2001;
18 (2): 176-181.
Banks MR, Farthing MJG.
Current management of Acute Diarrhoea. Prescriber 2001;
12(12):83-93.
Banks MR, Farthing MJG.
The Management of Acute Diarrhoea. Prescriber 2000;
11(4): 97-105
Case reports
Pasha Y, Banks M.
Medical mystery: an unusual cause of anaemia
Br J Hosp Med (Lond). 2010 Feb;71(2):113.
Pasha Y, Pickard L, Cohen P, Banks MR
An unusual endoscopic diagnosis for acute epigastric pain
Scand J Gastro 2008;43(9):1151-2.
Pasha Y, White WJ, Chew NS, Banks M.
The importance of never ignoring an unexplained metabolic acidosis. Incarcerated femoral hernia. QJM.
2008 Oct;101(10):825-6. Epub 2008 Aug 28.
Green L, Banks MR
HIV associated encephalopathy, a grey case. Int J STD’s AIDS.
1995; 6: 744
Original papers
Radiofrequency ablation for early oesophageal squamous neoplasia: Outcomes form United Kingdom registry.
Rehan J Haidry, Mohammed A Butt, Jason Dunn, Matthew Banks, Abhinav Gupta, Howard Smart, PradeepBhandari, Lesley Ann Smith, Robert Willert, Grant Fullarton, Morris John, Massimo Di Pietro, Ian Penman, Marco Novelli, Laurence B Lovat
World Journal of Gastroenterology 09/2013; 19(36):6011-6019. 2.47
Radiofrequency Ablation (Rfa) And Endoscopic Mucosal Resection For Dysplastic Barrett’s Esophagus And Early Esophageal Adenocarcinoma: Outcomes Of Uk National Halo Rfa Registry.
R J Haidry, J M Dunn, M A Butt, M Burnell, A Gupta, S Green, H Miah, H L Smart, P Bhandari, L Smith, R Willert, G Fullarton, M Di Pietro, C Gordon, I Penman, H Barr, P Patel, P Boger, N Kapoor, B Mahon, J Hoare, R Narayanasamy, D O’Toole, E Cheong, N C Direkze, Y Ang, M Novelli, M R Banks, L B Lovat
Gastroenterology. 2013 Mar 28.doi:pii: S0016-5085(13)00459-9. 10.1053/j.gastro.2013.03.045
Wallace MB, Crook JE, Saunders M, Lovat L, Coron E, Waxman I, Sharma P, Hwang JH, Banks M, DePreville M, Galmiche JP, Konda V, Diehl NN, Wolfsen HC. Multicenter, randomized, controlled trial of confocal laserendomicroscopy assessment of residual metaplasia after mucosal ablation or resection of GI neoplasia in Barrett’s esophagus. GastrointestEndosc. 2012 Sep;76(3):539-47.e1. doi: 10.1016/j.gie.2012.05.004. Epub 2012 Jun 28
Dunn JM, Mackenzie GD, Banks MR, Mosse CA, Haidry R, Green S, Thorpe S, Rodriguez-Justo M, Winstanley A, Novelli MR, Bown SG, Lovat LB. A randomised controlled trial of ALA vs. Photofrin photodynamic therapy for high-grade dysplasia arising in Barrett’s oesophagus. Lasers Med Sci. 2012 Jun 15.
Banks MR, Haidry R, Butt MA, Whitley L, Stein J, Langmead L, Bloom SL, O’Bichere A, McCartney S, Basherdas K, Rodriguez-Justo M, Lovat LB. High resolution colonoscopy in a bowel cancer screening program improves polyp detection. World J Gastroenterol. 2011 Oct 14;17(38):4308-13.
Dunn JM, Banks MB, Oukris D, McKenzie GD, Thorpe S, Winstanly A, Novelli MR, Bown S, Lovat LB. Radiofrequency ablation is an effective treatment for high grade dysplasia in Barrett’s esophagus after failed Photodynamic therapy – a case series. Endoscopy. 2011 Jul;43(7):627-30
Dunn JM, Mackenzie GD, Oukrif D, Mosse CA, Banks MR, Thorpe S, Sasieni P, Bown SG, Novelli MR, Rabinovitch PS, Lovat LB. Image cytometry accurately detects DNA ploidy abnormalities and predicts late relapse to high-grade dysplasia and adenocarcinoma in Barrett’s oesophagus following photodynamic therapy. Br J Cancer 2010 May 25;102(11):1608-17
Kent AJ, Graf B, Prasad P, Banks M, Feher M. Diabetes Treatments, Gastrointestinal Symptoms and lower Gastrointestinal Endoscopy. Br J Diabetes &Vasc Dis 2009; 9: 129
Banks MR, Farthing MJG, Robberecht P, Burleigh DE. Anti-secretory actions of a novel vasoactive intestinal polypeptide (VIP) antagonist. British J Pharmacol 2005; 144: 994-1001.
Banks MR, Golder M, Farthing MJG, Burleigh DE. Intracellular potentiation between two second messenger systems may contribute to cholera toxin-induced intestinal secretion in humans.GUT 2004;53:50-57
Mulcahy HE, Kelly P, Banks MR, Connor P, Patchet SE, Farthing MJG, Fairclough PD, Kumar PJ. Factors Associated with Tolerance to, and Discomfort with, Unsedated Diagnostic Gastroscopy.Scand J Gast 2001; 36: 1352-1357
Banks MR, Kumar PJ, Mulcahy HE. Pulse Oximetry saturation levels during routine unsedated diagnostic upper gastrointestinal endoscopy.Scand J Gast 2001; 36: 105-109.
Pollock RCG, Banks MR, Fairclough PD, Farthing MJG. Dilutionaldiarrhoea – underdiagnosed and over-investigated.Europ J GastHep. 2000; 12: 1-3
Rockall AG, Lamb GM, Banks MR, Barrett SP, Al-Kutoubi MA. A prospective study of bacteraemia and bacterial contamination rates of catheters and wires during angiography.JInterventRadiol 1997; 12; 107-111.
Directory:
Tags:

|
Directory:
Tags:

|
|
|

|
Directory:
Tags:
|
Directory:
Tags:
|
|
|

|
|
Directory:
Tags:

|
Directory:
Tags:
|
Directory:
Tags:
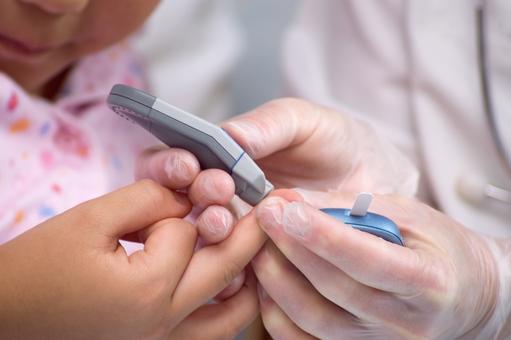
- A 2017 research project found that only 6 out of 18 FDA-approved blood glucose monitoring (BGM) systems tested were accurate
- Each day BGM systems are used by millions of people with diabetes to help them self-manage their condition, and avoid devastating and costly complications
- Thousands of similar smart devices support the prevention and self management of other chronic lifetime conditions, whose prevalence levels are high
- The increasing demand for healthcare, its escalating costs, and rapidly evolving technologies are driving the growth of such remote self-managed devices
- The most valuable aspect of such devices is the data they produce
- These data tend to be under valued and under utilized by healthcare providers
- This has created an opportunity for giant technology companies to enter the healthcare market with a plethora of smart devices and start utilizing the data they collect to enhance patient outcomes and lower costs
- Giant technology companies could dis-intermediate GPs and re-engineer primary care
Digital blood glucose monitors and the disruptive impact of giant tech companies on healthcare
A 2017 research project, which tested 18 FDA-approved digital blood glucose monitoring (BGM) systems, which are used daily by millions of people with diabetes to check the concentration of glucose in their blood, found that only 6 were accurate. The research, led by David Klonoff of the Diabetes Research Institute at San Mateo, California, was funded by Abbott Laboratories.
This Commentary describes both traditional and next-generation BGM systems, and Klonoff’s research. The Commentary suggests that BGM systems are just one part of a vast, global, rapidly growing market for consumer healthcare devices, and argues that the most valuable aspect of these devices is the data they collect. With some notable exceptions, healthcare professionals do not optimally utilize these data to enhance care and reduce costs. This has created for an opportunity for technology companies to enter the healthcare market and re-engineer primary care. The one thing, which might slow the march of giant technology companies into mainstream healthcare, is the privacy issue.
Traditional and next-generation BGM systems
Next generation BGM system
Abbott Laboratories Inc. markets a BGM system, which eliminates the need for routine finger pricks that are necessary when using traditional glucose monitors. Instead of finger pricks and strips, the BGM system, which measures interstitial fluid glucose levels, comprises a small sensor and a reader. An optional companion app for Android mobile devices is also available. The sensor is a few centimetres in diameter and is designed to stay in place for 10 days. It is applied to the skin, usually on the upper arm. A thin (0.4 mm), flexible and sterile fibre within the sensor is inserted in the skin to a depth of 5 mm. The fibre draws interstitial fluid from the muscle into the sensor, where glucose levels are automatically measured every minute and stored at 15-minute intervals for 8 hours. Glucose levels can be seen at any time by scanning the reader over the sensor. When scanned the sensor provides an answer immediately. It also shows an 8-hour history of your blood glucose levels, and a trend arrow showing the direction your glucose is heading. The device avoids the pain, and inconvenience caused by finger-prick sampling, which can deter people with diabetes from taking regular measurements. In the UK the system costs £58 for the reader, plus £58 for a disposable sensor, which must be replaced every 10 days and from November 2017 have been available on the NHS. Abbott Laboratories is a global NASDAQ traded US MedTech Company, with a market cap of US$86bn; annual revenues of US$21bn, and a diabetes care division, which produces annual revenues of some US$600m.
BGM systems used by Klonoff and his team for their research were acquired over-the-counter and independent of their manufacturers. All were tested according to a protocol developed by a panel of experts in BGM surveillance testing.
Klonoff’s research specified that for a BGM system to be compliant, a blood glucose value must be within 15% of a reference plasma value for a blood glucose >100 mg/dl, and within 15 mg/dl of a reference plasma value for a blood glucose approved” a BGM system had to pass all 3 trials. Only 6 out of 18 passed by achieving an overall compliance rate of 95% or higher.
|
|













